Coronavirus Vaccine: Pfizer Says It Won’t Seek Authorization Before Mid-November
4 min read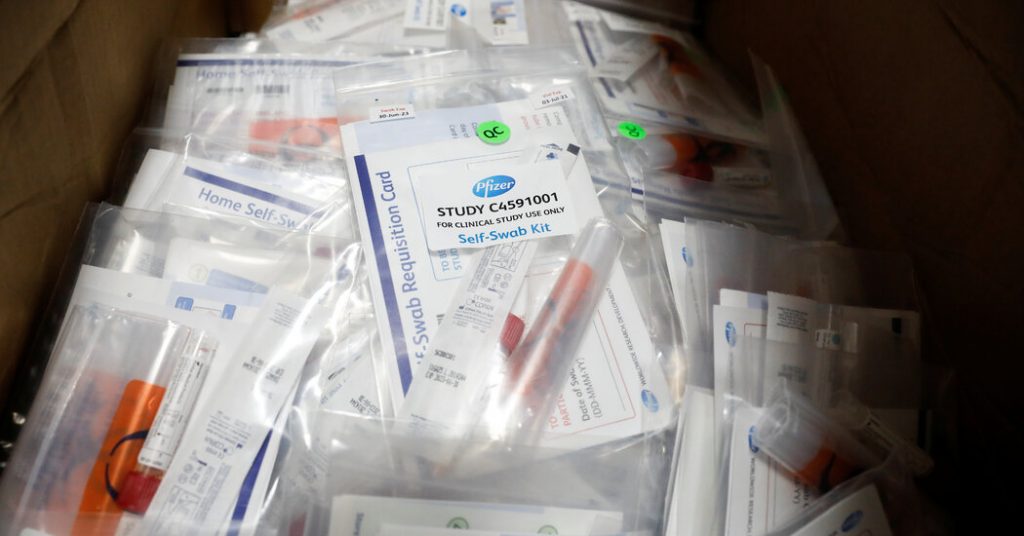
Stick to our reside protection of the coronavirus pandemic in this article.
The main govt of Pfizer said on Friday that the corporation would not apply for crisis authorization of its coronavirus vaccine ahead of the 3rd 7 days of November, ruling out President Trump’s assertion that a vaccine would be ready ahead of Election Working day on Nov. 3.
In a assertion posted to the enterprise web site, the main executive, Dr. Albert Bourla, stated that even though Pfizer could have preliminary quantities by the end of October about irrespective of whether the vaccine functions, it would even now have to have to acquire basic safety and producing facts that will extend the timeline to at minimum the 3rd week of November.
Shut watchers of the vaccine race had already recognised that Pfizer wouldn’t be capable to fulfill the prerequisites of the Foods and Drug Administration by the finish of this thirty day period. But Friday’s announcement signifies a shift in tone for the firm and its chief, who has consistently emphasised the thirty day period of Oct in interviews and community appearances.
In carrying out so, the corporation had aligned its messaging with that of the president, who has built no mystery of his wish for an permitted vaccine in advance of the election. He has even singled out the firm by identify and explained he had talked to Dr. Bourla, whom he named a “great male.”
Some experts applauded Pfizer’s announcement.
“This is good, genuinely fantastic,” mentioned Dr. Eric Topol, a medical trial professional at Scripps Analysis in San Diego who was just one of 60 community health officers and other folks in the healthcare group who signed a letter to Pfizer urging it not to hurry its vaccine.
Maintain up with Election 2020
He claimed company officials experienced assured him that a vaccine would most possible not be authorized before the election, but the letter Friday is “even extra sound about their not remaining aspect of any political machinations.”
Dr. Bourla has pushed back versus any recommendation that Pfizer’s vaccine timeline was politically inspired. In September, Pfizer was the driving force powering a pledge by 9 vaccine corporations to “stand with science” and not put ahead anything at all that experienced not been appropriately vetted. Earlier this thirty day period, he revealed an open up letter to workers that said he “would never succumb to political pressure” and expressing disappointment that “we uncover ourselves in the crucible of the U.S. presidential election.”
Pfizer is 1 of 4 organizations testing a coronavirus vaccine in late-stage scientific trials in the United States, and it has been the most intense in its timeline estimates. Moderna, AstraZeneca and Johnson & Johnson have claimed that afterwards in the yr is far more most likely, matching the predictions of federal wellness officials. (AstraZeneca and Johnson & Johnson’s trials have been paused for potential basic safety fears, which could further more hold off their outcomes.)
In interviews, Mr. Bourla has reported that he expects a “conclusive readout” by late October, with an application for crisis authorization that could be submitted “immediately.”
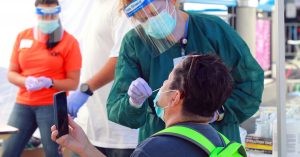
Pfizer’s demo of 44,000 volunteers assessments the vaccine by giving 1 group the vaccine, a further group the placebo, and ready till a selected quantity of men and women turn out to be contaminated with the virus. If considerably a lot more folks who received the placebo acquired contaminated, then the vaccine is regarded as to be efficient.
A organization spokeswoman reported final month that Pfizer would not be everywhere close to completion of its trial by the finish of Oct and that when Dr. Bourla had referred to a “conclusive readout,” he intended it was attainable the outdoors board of professionals checking the demo would have by that day identified promising signs that the vaccine operates.
In his assertion on Friday, Dr. Bourla acknowledged individuals timelines had been unsure. “Since we must wait for a selected quantity of scenarios to occur, this knowledge may arrive previously or later based mostly on modifications in the infection charges.”
He also claimed the firm would release the success of any choice by the outside the house panel — very good or terrible — within a few times of its final decision.
Dr. Bourla’s assertion arrived shortly following the F.D.A. published new guidelines detailing how the company would appraise a vaccine for emergency authorization, a document revealed right after months of stalling by the White House. The rules, which do not have the power of legislation, simply call for gathering complete basic safety information in the final phase of clinical trials ahead of an crisis authorization can be granted.
In a tweet on Oct. 6, Mr. Trump accused the F.D.A. of getting a political agenda with the suggestions, which he explained “make it extra difficult for them to velocity up vaccines for acceptance just before Election Working day.” Mr. Trump termed it a “political strike position.”
In a movie he posted from the White Household a day later, Mr. Trump claimed that a vaccine really should be accessible in advance of the election, “but frankly, the politics gets included.”
“They want to participate in their online games,” he mentioned. “It’s likely to be ideal immediately after the election.”
Jonathan Martin contributed reporting.